Abstract
Background: The pathogenesis of acquired aplastic anemia has been recognized as a multifaceted autoimmune bone marrow (BM) failure mainly attributed to the offsetting of functional T lymphocyte homeostasis. Although bulk genomic landscape or transcriptomic profiling have contributed a better understanding of recurrent cytogenetic abnormalities and immunologic cues associated with the onsets of hematopoietic destruction, the functional determinants underlying the complexity of heterogeneous T lymphocytes as well as their clinical significances remained to be exquisitely elucidated.
Methods: We rationally adopted single cell mass cytometry (also known as 'cytometry by time-of-flight' or CyTOF) from severe aplastic anemia (SAA) bone marrow mononuclear cells (BMMCs) at pre- or post-IST stages and healthy BMMCs to functionally define critical BM cellular compartments correlated with diseases onsets and therapeutic responses. Building on the functionally characterized composition in T-lineage compartment, we deeply validated the potential regulatory mechanisms underlying immune-based hematopoietic sabotage in aplastic anemia by single cell RNA sequencing, pinpointing indicative biomarkers correlated with clinical outcomes as well as feasible therapeutic targets in autoimmune diseases of similar mechanisms.
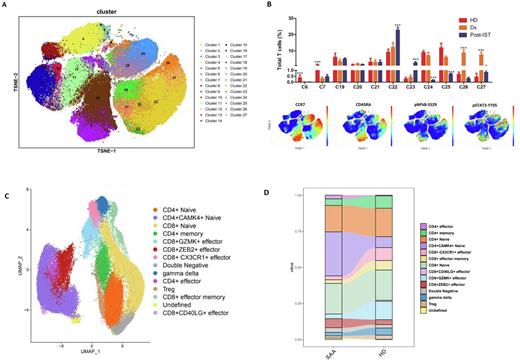
Results: Comprehensive profiling of BMMCs from 9 SAA samples at diagnosis (Dx-SAA), 3 SAA samples after immunosuppressive therapy (postIST-SAA), and 4 BMMCs from pediatric healthy donors (HD) with a predefined marker panel including critical surface antibodies (n = 31) and phosphorylated antibodies against key protein kinases (n = 11) partitioned the 27 clusters into 8 majorly functional categories, including stem/progenitor cells, granulocytes, erythrocytes, and immune subsets (monocytes(Mos), dendritic cells(DCs), B cells, T cells and NK cells). Of note, the proportion of all the non-immune subsets, and Mos and DCs in immune subsets were extremely decreased in Dx-SAA, whereas the fraction of T cells was significantly elevated in Dx-SAA, in contrast to both postIST-SAA and HD. Based on CyTOF signatures as well as flow cytometric validation on the potentially active T lineage subsets in SAA onsets, a subgroup of non-canonical CD4+ Naïve T cells with elevated phosphorylation of NFkB (pNFkBS529) and STAT3 (pSTAT3Y705) was speculated to be putatively associated with pathogenesis of SAA (Fig.1A-B).
To further clarify the precise roles of the distinct CD4+ naïve T cells in the occurrences of pediatric AA, we carried out high-throughput droplet-based 5'-single-cell RNA-seq (scRNA-seq) and paired T cell receptor sequencing (scTCR-seq) of isolated CD3+ BMMCs from the aforementioned five Dx-SAAs and two HDs. Among the 14 sub-clustered T-lineage cell types, the CD4+CAMK4+ naïve T cells with functional pathway such as Th17 differentiation (JAK3 andSTAT3), TCR pathway activation (ITK and ZAP70), and the epigenetic regulators (KDM6A and XIST) caught our attention, corresponding to the features from aforementioned CD4+ naïve T cells by CyTOF analysis (Fig. 1C-D). Concordant with this discovery, the phospho-flow cytometric analysis for STAT3pY705 and STAT3pY727 from CD4+ cells in BMMCs of SAA patients and HDs strongly implied the aberrant activation of JAK3/STAT3 pathway in Th17-polarized CD4+ naïve T cells among SAA onsets.
Conclusions: To our knowledge, our findings from a combination of single cell mass cytometry and single cell transcriptomic profiling have shed light on the functional roles of separated compartments of BM-infiltrating T cells in immune-mediated hematopoietic devastation in severe aplastic anemia. By tailoring scRNA-seq and CyTOF analytic tools to decipher the molecular determinants of autoimmunity, we innovatively identified the potential Th17-polarization with JAK3/STAT3 activation underlying the pathogenesis of CD4+CAMK4+ naïve T cells in SAA at single-cell transcriptomic and proteomic levels, warranting further investigation for targeted therapy with activated JAK3/STAT3 signaling in SAA.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal